New Clinical Trial in MDR-TB Care to Support Hardest to Treat Patients Globally
Q&A with Dr. Carole Mitnick, PIH’s co-principal investigator of endTB project

Tuberculosis is the most deadly infectious disease, killing more than 1.4 million people globally, most of them in resource-poor countries. Some forms of the disease are resistant to treatment and therefore can’t be cured using standard drugs. These multidrug (MDR)- and extensively drug resistant strains are particularly worrisome to patients and clinicians, and they are rapidly spreading across countries with the least resources to tackle them.
To meet this challenge, endTB was formed. The project, an international partnership among Partners In Health, Médecins Sans Frontières (MSF), and Interactive Research and Development (IRD) and funded by UNITAID, aims to find shorter, less toxic, and more effective treatments to MDR-TB. This will largely be achieved through the introduction of the first new TB drugs in more than 40 years, two clinical trials testing shorter regimens, and advocacy at national and global levels.
Launched earlier this year, the endTB-Q trial aims to establish the efficacy and safety of shortened, all-oral drug regimens in a subgroup of patients with MDR-TB, those with resistance to the most effective drug in second-line TB treatment. All this has moved forward while the endTB team has faced significant challenges due to the spread of COVID-19, another airborne infectious disease.

Dr. Carole Mitnick, endTB’s co-principal investigator, a professor of Global Health and Social Medicine at Harvard University, and senior TB researcher with PIH, explains why TB disproportionately affects the marginalized, discusses the importance of the endTB-Q trial, and shares how teams have adapted their work during the COVID-19 pandemic.
Why is TB more present in marginalized populations than in the general population?
TB transmits in enclosed, crowded spaces. Like COVID-19, TB transmission is airborne, usually after longer, sustained contact. Poor and marginalized people are more likely to live in crowded environments where they face increased risk of infection.
About 90% of those infected never get active disease, but nutritional deficiencies and other conditions like HIV, diabetes, and substance-use disorders increase vulnerability to developing active disease.
Why does endTB specifically focus on multidrug-resistant tuberculosis, or MDR-TB?
Like TB, MDR-TB is a disease that exploits marginalization, and disadvantage. It occurs commonly among people who suffer from poverty and other associated conditions and who live in countries that make tiny investments in health. These diseases do not inspire private industry to develop drugs; shareholders don’t see big opportunities for earnings in drugs developed for people and health systems that can’t pay. Consequently, drug development for MDR-TB treatment has lagged.
Even the recent advances in development of two new drugs have not been adequately resourced to optimize their use. So these 3 NGOs—MSF, PIH, IRD—had to take on this responsibility.
It’s in our DNA to try to increase access to the best possible treatments and improve their use. And, we believe that poor people with MDR-TB are just as deserving of high-quality evidence to inform their care as, say, rich people with cardiovascular disease.
So, we had to take this on. To make matters worse, many trials exclude patients with comorbidities. But, because they are real issues faced by people with MDR-TB, endTB includes patients with comorbidities.
The proportion of cases that are drug-resistant is growing. In some places, like Kazakhstan, the proportion of TB cases that are resistant is higher than 40%. The more resistant the TB, the more expensive to treat, the less likely people are to get adequate treatment.
PIH and endTB believe that all patients are deserving of treatment. We know that not treating these patients can have a really devastating impact on the population around them because drug-resistant TB is highly transmissible.
Can you explain more about endTB and where the project has been conducted?
The endTB project is a series of studies that focus on treatment for MDR-TB–the form of TB that’s resistant to rifampin, the most potent drug in the first-line regimens. First, we did an observational study to show that newer (bedaquiline and delamanid) and repurposed (linezolid and clofazimine) drugs could be used safely in effective long regimens for MDR-TB. Then, we launched a randomized, controlled clinical trial to optimize the use of these drugs in shorter, all-oral, simplified regimens for forms of MDR-TB that remain susceptible to fluoroquinolones, which has historically been the most potent drug in second-line treatment.
This trial, known as the endTB trial, is examining five experimental regimens. It’s unusual to test so many regimens at one time, because normally researchers are looking for “a best.” Instead, at endTB, we are trying to identify as many successful regimens as possible. And, the trial uses “adaptive randomization” to assign patients to various treatment arms. We use information learned through the course of the trial to influence the chance of a patient being assigned to each regimen. More patients will be assigned to the regimens that are more effective. This approach allows us to do this in much less time, with a fraction of the patients than if all five regimens were tested in separate trials
The trial is being run on every continent except Australia and Antarctica, with active sites in Peru, Lesotho, Kazakhstan, South Africa, Pakistan, and India.
The regimens we are testing have the following benefits: 1) They are shorter than those currently used, nine months instead 18+-months. 2) They are all-oral [pills] instead of requiring months of daily injections; they may cause fewer negative side effects. 3) They use fewer drugs, no more than five (compared to seven or more), which will make them easier for patients to take and for health systems to stock at points of delivery.

Tell us about the new clinical trial launched this year.
This year, in the middle of the worst public-health pandemic in a century, we launched the second trial in the project, called endTB-Q. This trial focuses on the patients who have a form of TB resistant to both fluoroquinolones and rifampin, which is the most difficult to treat.
What’s really groundbreaking about the endTB-Q trial is that it is focused on this especially vulnerable population. endTB-Q is the only trial that tests all-oral regimens specifically for this group using the gold standard of clinical research: a randomized, internally concurrently controlled trial. By the end of 2021, we expect to finish enrollment of 324 participants. We expect to have the results by 2023.
How essential are our partnerships with MSF and IRD to the success of endTB trials?
It is hard to put into words how important these partnerships are and how difficult this work would be to do on our own. Working with these powerhouse groups amplifies our impact.
Among the consortium partners, we share a common set of beliefs about the right to high-quality care, regardless of where people happened to be born in the world, their religion, color, ethnicity, political beliefs, socioeconomic status or how much their government chooses to spend on health care. In fact, we all deliberately try to reach those who are most likely to be left behind by other efforts.
How have you been adapting to the pandemic while lifting up a major trial?
COVID-19 has had an enormous impact on our study sites, patients, staff, and the TB programs in which they’re embedded. Services have been shut down in many places and TB patients are not able to get care and diagnosis. This has deeply worrying consequences for these patients, their families, and communities; multiple models have predicted enormous increases in TB incidence and mortality in coming years, with effects being greatest for MDR-TB.
In all the sites, there have been moments when health facilities were completely closed or no TB patients were coming to health facilities out of fear. We did face setbacks, like when our whole Kazakhstan team got sick with COVID-19 and had to suspend the study for a few weeks.
And, this has resulted in delays in trial enrollment.
Despite the pandemic, we had to maintain the services we had committed to the trial participants whom we had already enrolled, at the same time, finding a way to continue enrolling patients in the endTB-Q trial.
Our primary concern was to protect staff and participants from COVID-19 and make sure that participants got their treatment and safety monitoring. We also needed to keep enrolling new patients, knowing that endTB was one of the only sources of care for MDR-TB patients in our study settings.
We had to set up guidance for the local teams on how to manage the virus, their own safety, and participants’ safety, providing information on what kind of treatments are available. Obviously, all of the staff are getting PPE. In addition, we have tried to provide extra food to support people who need to isolate or quarantine.
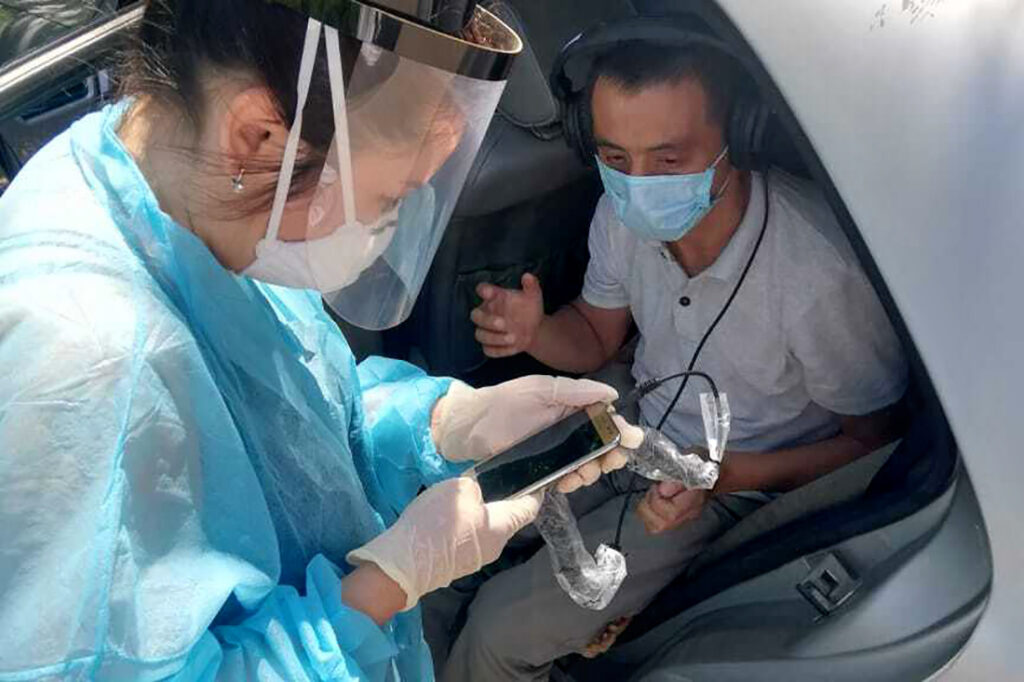
We’ve had to make some changes to the study and patient treatment, for example, moving visits out of health care facilities to either patients’ homes, fields, or even cars.
We started providing accommodation for patients in rare cases, private transportation to get participants to study visits, or to get study workers to participants’ homes. In some sites, we have expanded the hours of the appointment times, ensuring there are fewer people present at one time.
However, one advantage of having a multi-site trial is that the pandemic’s waves are hitting different areas at different times.
Overall, this experience has prepared us for inevitable future disruptions. During future pandemics or natural disasters, having shorter, less-toxic treatments for MDR-TB will be a huge win. It also highlights the importance of patience and long-term commitments to complex problems affecting poor people, which has always been a hallmark of PIH’s work. Even before the pandemic, we knew that being on the side of patients with MDR-TB—including through activities such as the endTB and endTB-Q trials—was a long-term proposition. COVID-19 has reinforced this lesson as well as our determination to ensure that funding, policy, and implementation partners remain committed and engaged for the long term.
In which ways does PIH help patients beyond the provision of TB medication and care?
We really try to imbue our research with the PIH model of care and support. Treatment is supported by a study worker or community member. Treatment supporters check in with participants to see how they are doing and to assess occurrence of adverse events. And, in both study and routine care, we provide nutritional support and transport. The trial adds the best level of clinical monitoring for disease. And, if study participants require hospitalization, they do not pay.
To counteract the effects of COVID-19, we have expanded all these supports and added more.
What kind of results have you achieved so far with endTB?
It’s too early to say about the trials. But, in the first study, the observational one, we provided new regimens to more than 2,800 patients in 17 countries. Some of them were very sick patients who had been treated unsuccessfully over and over again. This was a huge success both in scale of roll-out and outcomes.
We have seen very promising results; 85% have had great early response: at or six months after start of regimens containing the new drugs, their sputum culture results were negative for the bug that causes TB. These results have been observed in patients without serious complications as well as in those with diabetes and the more resistant forms of MDR-TB.
Regimens containing new and repurposed drugs are really, really promising, and they are promising both from an effectiveness standpoint and from a safety and tolerability standpoint. The new drugs can replace the old injectable ones that caused terrible side effects, like hearing loss and kidney problems, while the cardiotoxicity risk that many were worried about did not occur frequently.
Most importantly, we’ve successfully pushed back on the narrative that regimens containing new drugs can’t safely be introduced in settings with limited resources.

What kind of impact do you foresee if the endTB trials are successful? In other words, would you categorize this as a game-changer for global TB care?
The combination of cutting the duration of treatment by more than half and potentially reducing the toxicity burden to reduce demands on the health system is huge. With shorter, more user- and health-system friendly treatment, treatment can be made much more widely available.
The other thing that’s really transformative is that if it’s the right treatment, patients will stop transmitting sooner. We know that within hours or days of starting effective therapy, people stop transmitting and are no longer a risk to their family members and neighbours. If you get them on the right regimen sooner, that has enormous benefits for reducing the number of people infected overall.
At the moment, TB programs have to keep 13 to 15 drugs in stock to be able to offer treatment for all forms of TB. If the endTB trials establish safety and efficacy of, say, three novel regimens, programs would only need to purchase and manage five to seven drugs. More patients would be treated with the same drugs, which would give national TB programs more negotiating power with pharmaceutical companies.
Article originally published on pih.org



